Abstract
Cognitive deficits have been observed in children and adults with sickle cell disease (SCD), with and without cerebral infarcts. Risk factors include systemic inflammation, neuroinflammation, and severe anemia, and sociological risk factors such as parental education level and income. Additionally, there is a reported relationship between plasma cytokine levels and cognitive function in children with SCD, suggesting a link between inflammation and cognitive deficit in SCD. This study aims to examine the mechanism underlying the development of cognitive deficit in SCD with exposure to social stress, by using the repeat social defeat (RSD) paradigm in sickle cell mice. We hypothesized that neuroinflammation is a major underlying mechanism mediating the relationship between social stress and development of cognitive deficit in SCD. RSD was done by introducing a male intruder mouse (an aggressor) into an established cage of 3, 6 months old male sickle cell or control mice, every day, for two hours, for six consecutive days. Age and sex-matched control (sickle cell and non-sickle cell mice) cages were set up but without aggressor mice. On the seventh day, mice were tested for cognitive/behavioral deficit using novel object recognition (NOR) and fear conditioning (FC) test paradigms. Except for the aggressors, all mice used were Townes humanized sickle cell (SS) and control (AA) mice. To test the hypothesis that neuroinflammation is an underlying mechanism, a second cohort of SS and AA nice were randomly assigned to oral (in drinking water) minocycline treatment (90mg/kg) or placebo (drinking water). Mice within treatment arms were randomly assigned to RSD exposure or no RSD exposure. Minocycline treatment was started one day prior to the day of commencing RSD and terminated on the same day as the final RSD session and the dose was kept constant by adjusting the amount administered daily, using the water consumption from the previous day. Cognitive/behavioral testing was performed as before, starting the next day after day 6 of RSD and day 7 of treatment. Mice were sacrificed 1-2 days after completion of behavioral testing and brains were extracted for histological analysis. Cellular evidence of neuroinflammation in the hippocampus/dentate gyrus was determined using immunohistochemistry to quantify peripheral immune cell infiltrates (CD45+ (bone marrow-derived microglia), CD3+ (T-cell density), B220+ (B-cell density), and Iba1+ (activated microglia). We used Golgi-Cox impregnation to quantify spine density and maturity in cortical and hippocampal pyramidal neurons.
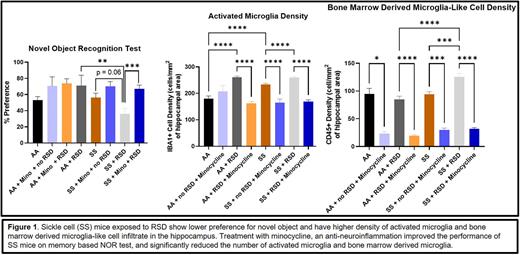
There were no differences between 6 months old AA and SS with regards to percent preference on NOR test (53.1 ± 4.4% vs. 56.1 ± 5.6%). However, SS mice exposed to RSD, had a lower percent preference (36.1 ± 6.7%, p = 0.06) for the novel object compared to SS mice not exposed to RSD (56.1 ± 5.6%), indicating the presence of a memory deficit in SS mice exposed to RSD. The SS mice exposed to RSD had worse performance on contextual FC test (p = 0.001), indicated by a lower percent freezing compared with SS mice not exposed to RSD. Additionally, SS mice exposed to RSD also had a higher density of activated microglia (260.2 ± 3.7 vs. 233.6 ± 4.0; p ≤ 0.0001) and bone marrow derived microglia (125.4 ± 6.2 vs. 93.7 ± 5.0; p = 0.0004) in the dentate gyrus, compared to SS mice not exposed to RSD, suggesting increased neuroinflammation after RSD exposure. There were no differences in B or T cell density. SS mice treated with minocycline during RSD exposure showed improvement in memory based on NOR (67.1 ± 4.5% vs 36.1 ± 6.7%, p = 0.0007) and contextual FC (p = 0.01) tests compared to SS mice exposed to RSD and not treated with minocycline. The treated mice had fewer activated microglia (168.4 ± 6.7 vs. 260.2 ± 3.7; p ≤ 0.0001) and bone marrow derived microglia (31.79 ± 2.2 vs. 125.4 ± 6.2; p≤0.0001) in the dentate gyrus compared to the non-treated SS mice exposed to RSD. Additionally, the treated SS mice exposed to RSD also showed increases in neuron progenitor stem cells density improvement in neurogenesis and a decrease in astrogliosis in granular zone of the dentate gyrus, compared to the SS mice exposed to RSD without minocycline treatment.
We demonstrate for the first time that neuroinflammation is one of the underlying mechanisms of social stress related cognitive impairment in mice and may be applicable to children with SCD and might be treatable using anti-neuroinflammatory drugs.
Disclosures
Archer:Forma Therapeutics: Research Funding; Global Blood Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal